Introduction
JW Creagene is a specialized company for manufacturing and developing patient customized cell therapy products, dendritic cell vaccines targeting various types of solid cancers and autoimmune diseases. All cell therapy products in JW Creagene are manufacturing in our GMP facility, which was approved by KFDA in 2007, under the well-defined guidelines and QC protocols. Now, JW Creagene has launched a CMO service for researchers and companies needing GMP level manufacturing of cell therapy products. Based on over 12 years of skills and experiences running GMP facility, JW Creagene’s CMO service will provide an ideal solution for managing/manufacturing GMP grade cell therapy products for customers. JW Creagene’s CMO offers the following services:
List of CMO service
- Manufacturing cell therapy products in a GMP facility for research and clinical trials.
- Large scale production of cell therapy products.
- Leasing GMP facility for manufacturing cell therapy products.
List of QC tests for CMO service
- Sterility test
- Mycoplasma test (PCR)
- Endotoxin test
- Identity test (cellular phenotype assay by flow cytometer)
- Efficiency test (functional assay using ELISA
- Adventitious viruses
Overview of JW Creagene’s GMP facility (approved by MFDS in 2007)

Manufacturing process of cell therapy products in GMP facility

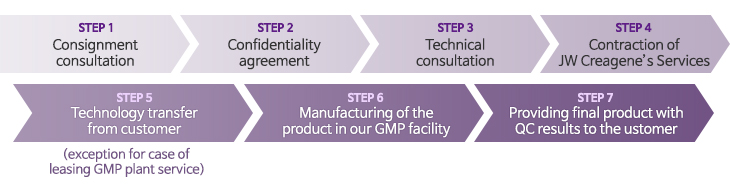
Application procedure of CMO service
T. +82 31-737-3300