Quality Control
For the Best Quality!!
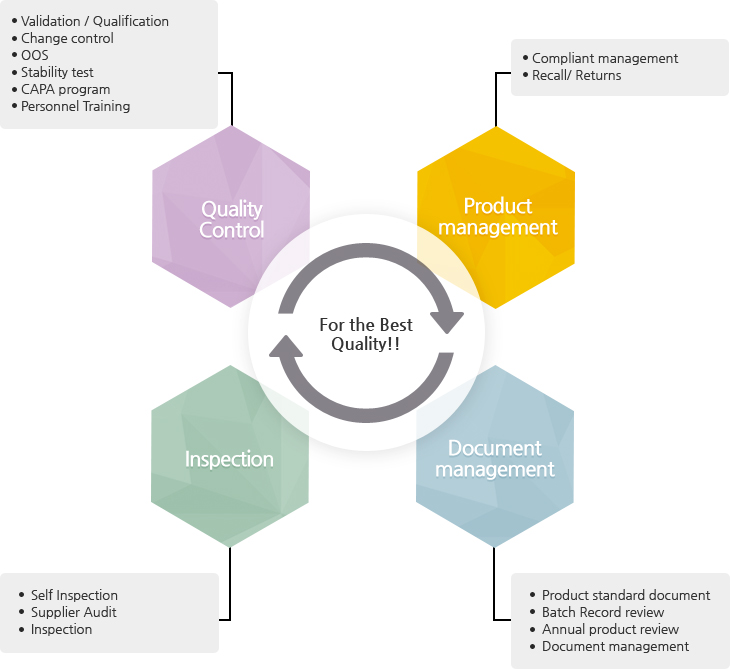
Quality Control
- Validation / Qualification
- Change control
- OOS
- Stability test
- CAPA program
- Personnel Training
Product management
- Complaint management
- Recall
- Returns
Inspection
- Self Inspection
- Supplier Audit
- Inspection
Document management
- Product standard document
- Batch Record review
- Annual product review
- Document management
Manufacturing
Manufacturing facilities customized to the patients
The manufacturing facility of JW CreaGene dedicated to the dendritic cell therapeutics and customized to the patient was certified as an exclusive Good Manufacturing Practice (GMP) plant for biomedical products. It is equipped with 5 independent aseptic manufacturing rooms, 3 aseptic test rooms used exclusively for quality control and auxiliary facilities. It is designed with patient-customized system for the entire processes from manufacturing of complete product to shipment through aseptic procedures.
Application of KGMP cleanliness
Cleanliness is maintained at the work place in accordance with the regulations of KGMP. We are implementing real-time environmental monitoring for the districts for which high level of cleanliness must be maintained in order to manufacture safe products. In addition, we have installed the system for monitoring of temperature, humidity and differential pressure in real time in each of the work places.
Highly experienced workers
Given the characteristics of the therapeutics customized to the patients, all the workers in the area of manufacturing and quality control have majored in microbiology or immunology related areas, and are equipped with high level of understanding of the products as well as stable product manufacturing capabilities through prolonged period of practical experiences.
Relevant facilities